国家自然科学基金委员会官网报道我科曹炬教授团队重要研究成果
发布时间:2024.03.04
字号:
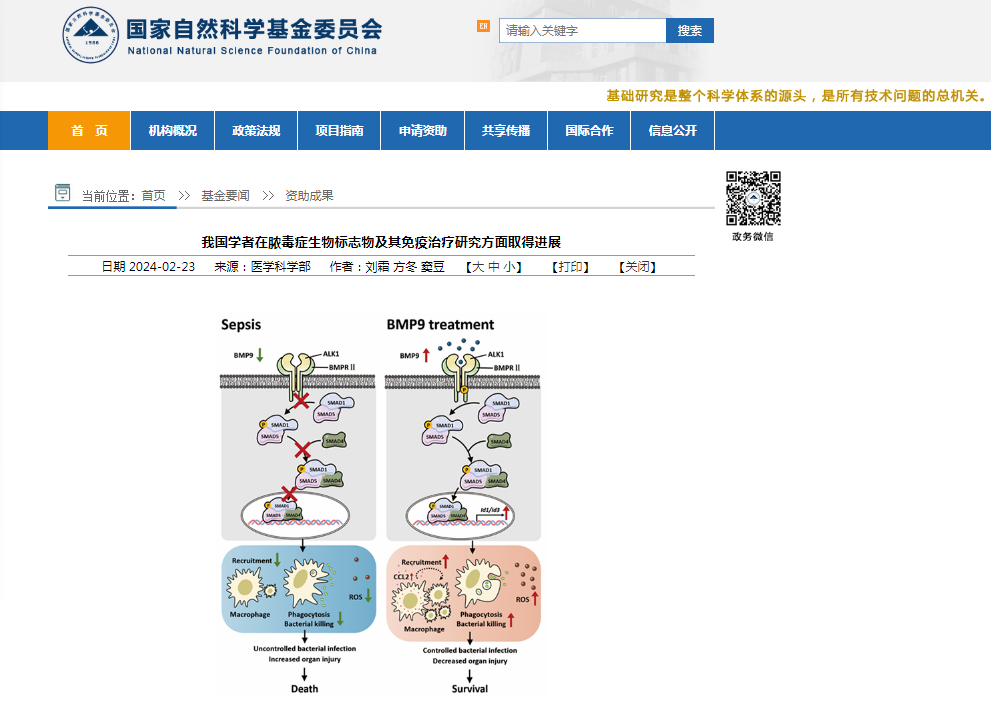

据悉,今年1月31日,曹炬教授团队的研究成果以《骨形态发生蛋白9是脓毒症的候选预后生物标志物和宿主定向治疗靶点》为题,在Science Translational Medicine杂志发表后,立即被JAMA正刊(IF=120.7)作为杂志当期要闻报道,同时,被期刊Nature Reviews Drug Discovery(IF=120.1)作为专题进行全篇亮点述评,凸显了国际医学顶级期刊对重医附一院该项重要科研成果的高度认可。据了解,《科技日报》《健康报》和《重庆日报》等媒体也对这一研究成果进行了报道。


当前,国家自然科学基金申报工作正在进行中。国家自然科学基金委员会官网、JAMA正刊和Nature子刊对重医附一院科研成果的报道和述评对医院的科技工作给予了极大的鼓舞,期待全院科研工作者在“科技强院”战略的指引下,提升科技创新能力,推动医院高质量发展,为服务人民健康提供坚实保障。
原文链接:
https://www.science.org/doi/10.1126/scitranslmed.adi3275
Edito’s summary
Abstract
SIGN UP FOR THE SCIENCEADVISER NEWSLETTER
INTRODUCTION
RESULTS
Characteristics of the human participants
Sepsis results in reduced serum BMP9 concentrations in patients
BMP9 concentrations predict survival in the patients with sepsis
BMP9 treatment improves outcomes in a murine sepsis model
BMP9 increases CCL2 but decreases IL-6 concentrations in experimental sepsis
Inhibition of endogenous BMP9 increases mortality and decreases bacterial clearance in experimental sepsis
BMP9 protects mice against sepsis by promoting macrophage recruitment and activation
BMP9 protects against experimental sepsis through CCL2 production
BMP9 can directly enhance bacterial phagocytosis and killing by macrophages
ALK1 signaling in macrophages plays a critical role in BMP9-induced protection against experimental sepsis
BMP9 promotes antibacterial functions of macrophages through the Smad1/5 pathway
BMP9 enhances antibacterial functions of human monocytes and monocyte-derived macrophages through the ALK1/ Smad1/5 signaling pathway
DISCUSSION
MATERIALS AND METHODS
Study design
Statistical analysis
Acknowledgments
向下滑动查看论文全文

曹炬 研究员








